Canine Leptospirosis: Treatment and Control
Leptospirosis is a disease with a great impact not only on the health of pets, but also on humans, since it is a zoonotic disease with worldwide distribution. Canine leptospirosis is a disease that can be acquired in both rural and urban environments, so the risk of exposure is latent.
Etiology
Canine leptospirosis is caused by bacteria of the Leptospiraceae family and Leptospira genus. They are Gram-negative and obligate aerobes with a spirochete shape (Figure 1) where a lipopolysaccharide membrane constitutes the main antigenic element (1). Additionally, the great mobility and ability to invade tissues through their adhesion proteins play an important role in determining the virulence of the bacteria (2).
Currently, they are classified into 64 different genome-species, grouped into two main clades: “Saprophytes” (organisms found in the natural environment) and “Pathogens” (which contains all the species responsible for infections in humans and animals, as well as environmental species of unknown virulence). Within these clades there are two subclades, the pathogenic subclades P1 (the old group of pathogens with 19 species) and P2 (the old group known as intermediate, with 20 species) and the saprophytic S1 and S2 (28 species) (3).
There is an older classification system based on serology, with approximately 300 serovars of pathogenic Leptospira grouped into 32 serogroups. Some of these serovars are found in more than one species of Leptospira. As a result, by convention, isolates are identified by both species and serovar. This serovar classification is the one commonly used in clinical practice and in the application of vaccines. The serovars most commonly found in dogs are Canicola, Icterohaemorrhagiae, Pomona, Bratislava and Grippotyphosa (4).
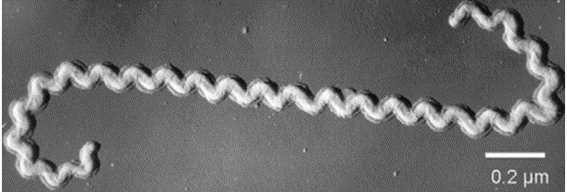
Figure 1: Electron microscopy of Leptospira spp.
Source: Picardeau. Diagnosis and epidemiology of leptospirosis (2013).
Epidemiology
Leptospirosis is prevalent mainly in geographic regions with a high level of annual rainfall and warm climates. However, other factors such as host exposure and the presence of domestic and wild animals influence the geographic distribution of the disease (4).
Infected animals play the role of reservoirs of leptospires, especially rodents, since, despite being infected, they do not usually present the disease, so the bacteria can remain in their organisms for months, years or a lifetime while they spread the bacteria (5).
Exposed animals become infected by contact of mucous membranes or skin wounds with infected urine or by contact of these with soil, water, food or bedding contaminated with infected urine. Although leptospira does not replicate in water, it can remain viable for weeks or months in urine. Transmission can also occur through bites, ingestion of infected tissues, and by venereal and placental routes (4).
Leptospira spp. adapts best to temperatures around 30ºC while ultraviolet radiation and freezing inactivate it. Canine leptospirosis affects dogs of any age, breed and sex. However, dogs living in areas where wild animals have contact with homes or where there are a large number of rodents and dogs working outdoors are at greater risk of infection (4).
Pathogenesis
The incubation period of canine leptospirosis normally lasts between 5 to 15 days, where the bacteria enter through mucous membranes or wounds and travel to the bloodstream where they multiply rapidly (bacteremia) from day 1 (4), then spread to target organs including the kidneys, lungs and liver (6).
In some animals with an adequate and high antibody titer the infection may be subclinical, so they do not show signs. Other individuals may present moderate signs with a short-term leptospirosis that will then be eliminated by the kidneys (6). While in certain cases the bacteria can persist and replicate in the tubular cells of the animal’s kidney, causing chronic elimination of leptospires in the urine for days, months or years (7).
In those animals sensitive to infection, there may be damage to the endothelium as a result of the multiplication of the organism in the bloodstream, which leads to ischemia in different organs such as the kidneys, lungs or liver. Neutrophils and platelets are stimulated by lipopolysaccharides in the outer membrane of leptospires, contributing to inflammation and coagulation disorders, as well as kidney and liver damage. Likewise, meningitis may occur if leptospires enter the nervous system or cerebrospinal fluid during the acute phase (7).
Clinical signs
Canine leptospirosis manifests with signs such as vasculitis, acute kidney injury and/or liver injury that may vary depending on the serovar and the host’s immune response. Fever, vomiting, abdominal or lumbar pain, polydipsia or polyuria, dehydration, pulmonary hemorrhage, uveitis, myositis, and reproductive failure may also occur (8).
Among the findings in serum biochemistry, the vast majority of cases include azotemia and hyperphosphatemia indicating renal injury. Other common findings are increased serum liver enzyme activity, hypoalbuminemia, and creatine kinase (8).
Dogs with chronic leptospirosis may have chronic hepatitis or liver fibrosis with signs that may include anorexia, weight loss, ascites, hepatic encephalopathy, and jaundice (9).
Diagnosis
In clinical practice, dogs that develop signs of acute renal failure or are jaundiced should be considered suspected cases of leptospirosis until a definitive diagnosis is made, particularly if they have not been vaccinated and are exposed to the bacteria (9).
Abdominal ultrasound can show changes related to renal injury that may include increased cortical echogenicity, enlarged kidney, mild pyelectasis, and perirenal effusion, a common finding in dogs with leptospirosis. Other findings that may be seen include hepatic changes such as hypoechoic parenchyma, hepatomegaly, and evidence of biliary sludge (8).
Bacterial culture allows the detection of leptospires, but is technically complicated to perform because it requires a special growth medium containing vitamin B and long-chain fatty acids. In addition, the culture time to identify bacterial growth can take several months, so it has little clinical utility and its use is more useful in epidemiological studies (8).
Molecular tests such as PCR can detect leptospiral DNA before the serological response to infection develops, making it a very useful test for diagnosing the early stages of the course of the disease (8).
The MAT test has been used for years as a reference method for diagnosing acute and convalescent cases of canine leptospirosis. False negatives may occur during the first week of infection, since in a single sample in acute infection the MAT test may have a sensitivity of 50%, so paired samples can be performed to raise this percentage to nearly 100%, with a specificity ranging from 70 to 100% (8).
Treatment
Early diagnosis is important to carry out a specific treatment and ensure recovery. Treatment should be started as soon as possible, preferably before the fifth day after the appearance of the first signs (8). However, treatment should not be delayed while waiting for the results of diagnostic tests (4).
Control and prevention
It is important to limit the dog’s access to potential sources of infection such as swampy areas and stagnant waters, as well as to minimize contact with wild animals by using fences and adequate rodent control (4).
Vaccines containing the serovars Icterohaemorrhagiae, Canicola, Grippotyphosa and Pomona are currently available. These vaccines effectively prevent the development of the disease as a result of exposure to the bacteria and the dissemination of the serovars included in the vaccine (4).
Immunity
Annual or semi-annual vaccination (depending on the type of vaccine) with 4-serovar vaccines is recommended for all dogs as it is considered part of a basic vaccination protocol. This is because dogs in both urban and rural areas are exposed to infection and developing the disease (4).
Vaccines should be administered annually to all dogs starting at 12 weeks of age, regardless of breed, as it can be serious or fatal despite treatment and exposure can occur regardless of age, and it is a zoonotic disease (10).
There is not much evidence about recurrent leptospirosis in dogs after appropriate treatment. However, vaccination is recommended as soon as possible after recovery from the disease as these dogs are at risk of exposure to the same or other serogroups, and it is unknown whether or not lifelong immunity results from natural disease. Further studies are needed to establish the true duration of immunity and the degree of cross-protection between specific serovars after natural infection in dogs (10).
Conclusions
Canine leptospirosis is a disease that has serious consequences for the dog’s health, and can in some cases cause kidney damage that persists even in dogs that have recovered. Because of this, treatment should be carried out as soon as possible, so an adequate and rapid diagnosis is essential.
Vaccination is very important in order to protect the dog from the clinical signs of the disease, so it must be included in all canine vaccination protocols. Likewise, access to sources of infection such as stagnant water must be restricted and the entry of rodents must be avoided.


